I. PROTOCOL: INDICATIONS-BASED PULMONARY DIAGNOSTICS: INITIAL SUBJECT EVALUATION
II. PURPOSE: To maintain continuity and assure appropriateness of physician orders for pulmonary diagnostic testing. The Pulmonary Diagnostic Technologist (PDT) will assess each request for pulmonary diagnostic testing, with regard to diagnosis, past medical history, or subject symptoms. If the diagnosis or past medical history are sound, the PDT will proceed with testing according to the “Assessment of Physician Request for Pulmonary Diagnostic Testing” Protocol. Otherwise, the “Initial Subject Evaluation” protocol will be initiated.
III. PATIENT TYPE: All patients referred, for testing, to the Pulmonary Diagnostic Services.
IV. TESTING AREA: Pulmonary Diagnostic Laboratories (adult and pediatric sites) and inpatient areas (bedside assessment).
V. EQUIPMENT AND FORMS NEEDED:
1. Calibrated (with verified calibration) pulmonary diagnostic equipment: Spirometer, Helium, Nitrogen and Carbon Monoxide analyzers, total body plethysmograph, pH-Blood Gas Analyzer and Hemoximeter, CPET equipment (including analyzers), Bronchoprovocation and related equipment for Respiratory Muscle Forces
2. Physician order (written, electronically generated, verbal), or “Request for Pulmonary Diagnostic Testing”
3. Testing medication(s)
4. TDPs for “Assessment of Request for Pulmonary Diagnostic Testing” and “Initial Subject Evaluation”, along with corresponding decision flow-charts.
VI. Overview:
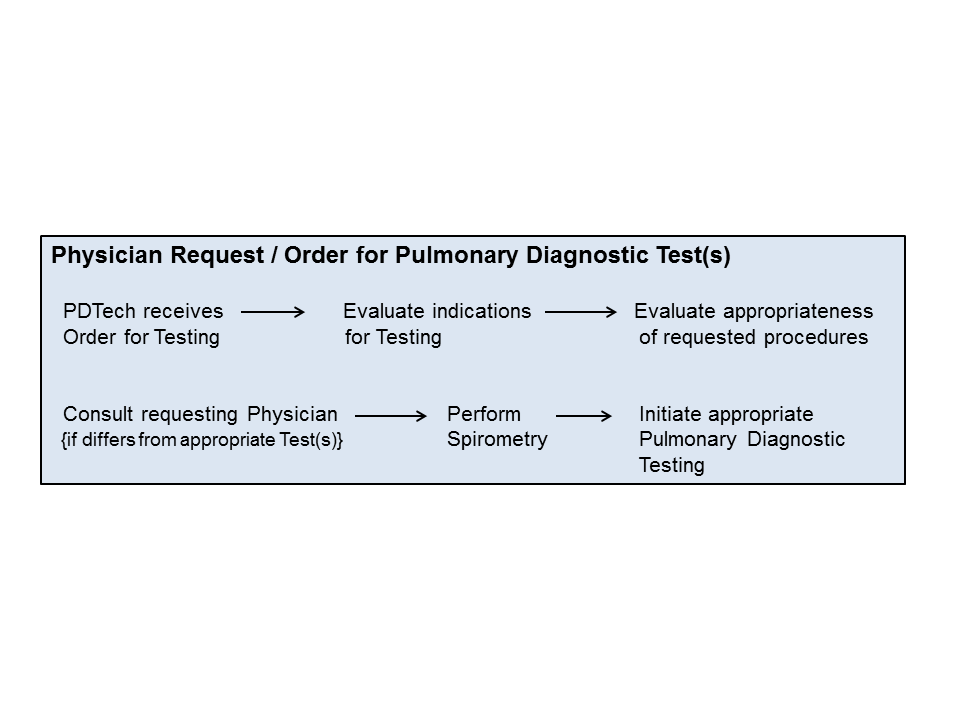
VII. PROTOCOL: The following guidelines will be followed when assessing appropriateness of physician requests for Pulmonary Diagnostic testing and are indicated by the PDT Assessment Protocol.
1. The following conditions are accepted indications for Pulmonary Diagnostic Testing1:
To determine
A. the presence and extent of lung disease or abnormality of lung function
B. the extent of disability due to abnormal lung function
C. the type and progression of the disease or lesion
D. a course of therapy in treatment of the particular lesion.
2. Pulmonary Diagnostic Tests available include (but are not limited to):
A. Spirometry with
1) evaluation of acute response to beta-agonist if airflow limitation is present,
2) maximal, forced inspiratory limb if upper airway dysfunction suspected (Flow-Volume Loop)
B. Maximum Voluntary Ventilation (MVV)
C. Lung Volumes (LV): Plethysmographic or gas dilution
1) with evaluation of acute response to beta-agonist if airflow limitation is present
D. Airway Mechanics (Raw, Gaw, sGaw)
1) with evaluation of acute response to beta-agonist if airflow limitation is present
E, Intrapulmonic Gas Distribution (SBN2, MBN2WO, HMT)
F. Arterial Blood Gases [ABGs] (includes Hemoximetry)
G. Respiratory Muscle Forces (RMF): AKA Maximal Occluding Pressure measurements
1) If Lung Volumes performed: MEP at FRC & TLC, MIP at FRC & RV
2) Otherwise MIP & MEP only
H. Cardiopulmonary Exercise Test (CPET)
I. Bronchial Provocation (Bronchoprovocation):
1) Non-specific airway challenge utilizing Methacholine Chloride
2) Exercise-induced: using treadmill or bicycle ergometer, with or without frigid air inhalation.
3. Baseline spirometry will be initially performed to assess the presence and degree of airflow limitation.
A. Spirometry indications include the need for
1. detecting the presence or absence of lung dysfunction suggested by history or physical indications (e.g., age, smoking history, family history of lung disease, cough) and/or the presence of other abnormal diagnostic tests (e.g., chest x-ray, arterial blood gases)
2. quantify the severity of know lung disease
3. assessing the change in lung function over time, or following administration of, or change of, therapy
4. assessing the potential effects, or response to, environmental exposure
5. assessing the risk for surgical procedures known to affect lung function
6. assessment of impairment and disability (pre-employment evaluations)
4. Selection of additional pulmonary diagnostic tests will be based upon the following procedure specific indications:
A. Spirometry with Bronchodilator (acute response assessment)
The need to assess
1, reversible airflow limitation: based upon spirometric evidence of
a. decreased FEV1 / FVC ratio (< predicted)
b. %predicted FEF25-75 / %predicted FVC < 0.8
c. decreased FEF50% and/or decreased FEF75% with normal PEF and/or FEF25%
2. evidence of central and/or peripheral airways disease
a. increased Raw (> 2.0 cm H2O/L/sec in adults)
b. decreased sGaw (< 0.17 L/sec/cm H2O/L)
3. reversible air-trapping evidenced by
a. increased RV/TLC ratio
B. Maximum Voluntary Ventilation (MVV)
To be employed
1. as a compliance indicator (e.g., post-operatively)
2. to assess respiratory muscle endurance (MVV < FEV1 X 35 with apparent maximal subject effort)
3. as a predictor of exercise VEMAX
4. to assess airway hyperreactivity
C. Static Lung Volumes
The need to
1. differentiate between obstructive and restrictive disease patterns3
a. VC and/or FVC < 80% predicted and/or < LLN; FVC with/without evidence of airflow limitation
2. rule out and/or quantify trapped gas6
a. increased RV/TLC ratio
3. assess response to therapeutic intervention (e.g., respiratory medications, radiation, transplantation, chemotherapy, lobectomy)3
4. quantify the presence and amount of unventilated lung (Open- or Closed-Circuit vs Plethysmographic methods)
5. assess effects of chronic disease (e.g., sarcoidosis, rheumatoid and uremic lung)
6. make preoperative assesments3 for upper abdominal or thoracic procedures
7. evaluate pulmonary disability
8. aid in the interpretation of other lung function tests2
D. Airway Mechanics (Raw, Gaw, sGaw)
The need to
1. differentiate types of obstructive patterns having similar spirometric configuration
2. differentiate sites of airway obstruction
a. central vs peripheral airway components
3. assess response to therapeutic intervention
a. bronchodilator (acute or chronic)
b. anti-inflammatory medications (e.g., steroids)
4. assess the magnitude of response to a bronchoprovocative agent
E. Diffusing Capacity4
1. Evaluation and follow-up of parenchymal diseases associated with dusts (e.g., asbestos) or drug reactions (e.g., amiodarone) or related to sarcoidosis;
2. Evaluation and follow-up of emphysema and cystic fibrosis;
3. Differentiating among chronic bronchitis, emphysema and asthma in subjects presenting with airflow limitation;
4. Evaluation of pulmonary involvement in systemic diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus);
5. Evaluation of cardiovascular disease (e.g., primary pulmonary hypertension, pulmonary edema, acute or chronic thromboembolism);
6. Prediction of arterial desaturation during exercise on subjects with COLD;
7. Evaluation and quantification of disability associated with interstitial lung disease;
8. Evaluation of the effects of chemotherapy agents, or other drugs known to induce pulmonary dysfunction;
9. Evaluation of hemorrhagic disorders.
F. Arterial Blood Gas (ABG) Analysis5
The need to
1. evaluate the adequacy of a subject’s ventilatory (PaCO2). Acid-base (pH and PaCO2), and/or oxygenation (PaO2 and O2Hb) status, and the oxygen-carrying capacity (PaO2, O2Hb, tHb and dyshemoglobin saturations) and intrapulmonary shunt (QSP/QT).
2. quantitate the response to therapeutic intervention (e.g., supplemental oxygen administration, invasive and/or non-invasive ventilatory support) and/or diagnostic evaluation (e.g., exercise desaturation).
3, to monitor severity and progression of documented disease processes.
G. Maximal Occluding Pressures (lung volume adjusted)
The need to assess
1. the presence and magnitude of respiratory muscle weakness
a. acute and chronic
2. the effect(s) of lung hyperinflation
3. response to therapeutic intervention
a. nocturnal ventilation
b. inspiratory muscle training
c. medications
d. nutrition
H. Bronchial Provocation7
The need to
1. diagnose or to confirm a diagnosis of airway hyperreactivity
2. follow changes in airway hyperresponsiveness
3. document the severity of airway hyperresponsiveness
4. determine who is at risk in the military or workplace
5. establish a control or baseline prior to a series of environmental or occupational exposures
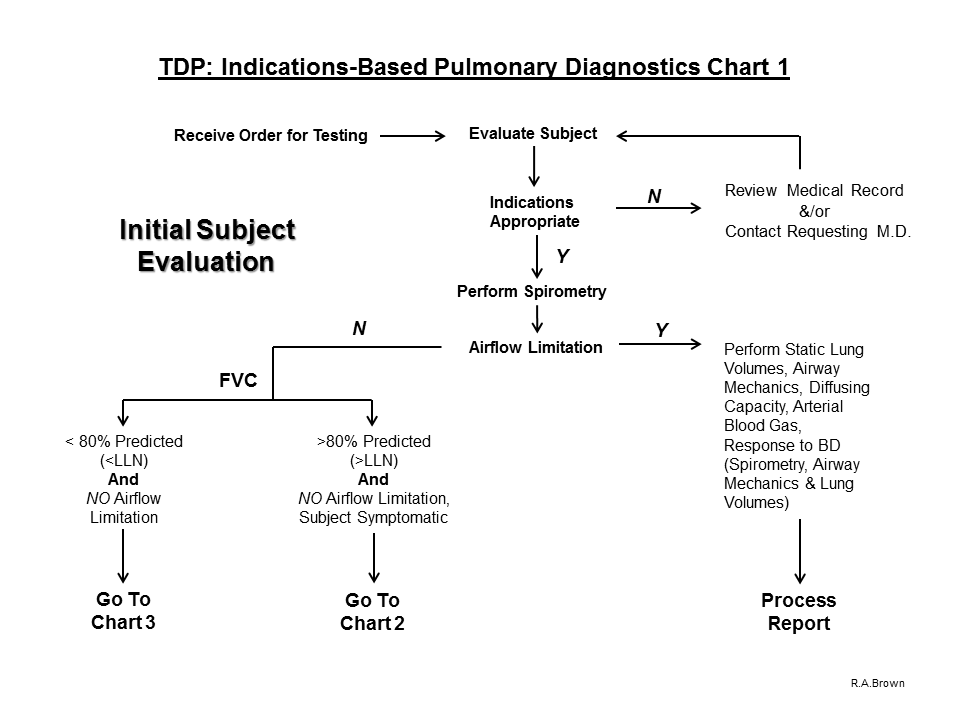
5. If airflow limitation is present on baseline Spirometry, the following Pulmonary Diagnostic tests are indicated:
A. Static Lung Volumes to evaluate air-trapping
B. Airway Mechanics to evaluate the site of airflow limitation
C. Diffusing Capacity to differentiate the type of ventilatory defect
D. Arterial Blood Gas Analysis to assess ventilation, oxygenation and acid-base status
E. Response to Bronchodilator (includes Spirometry, Airway Mechanics and Static Lung Volumes)
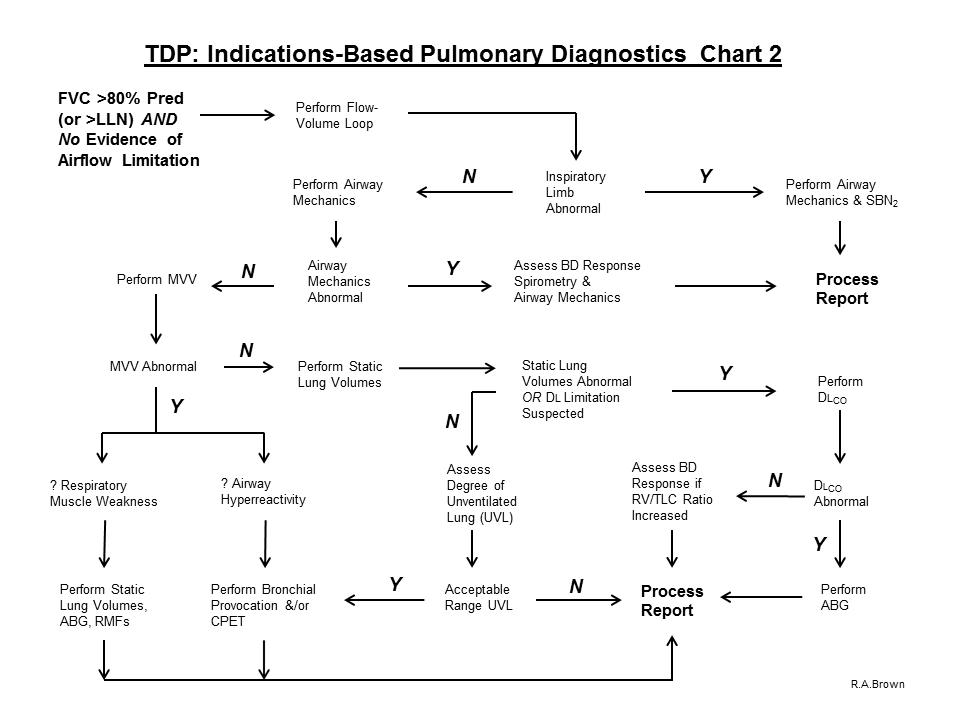
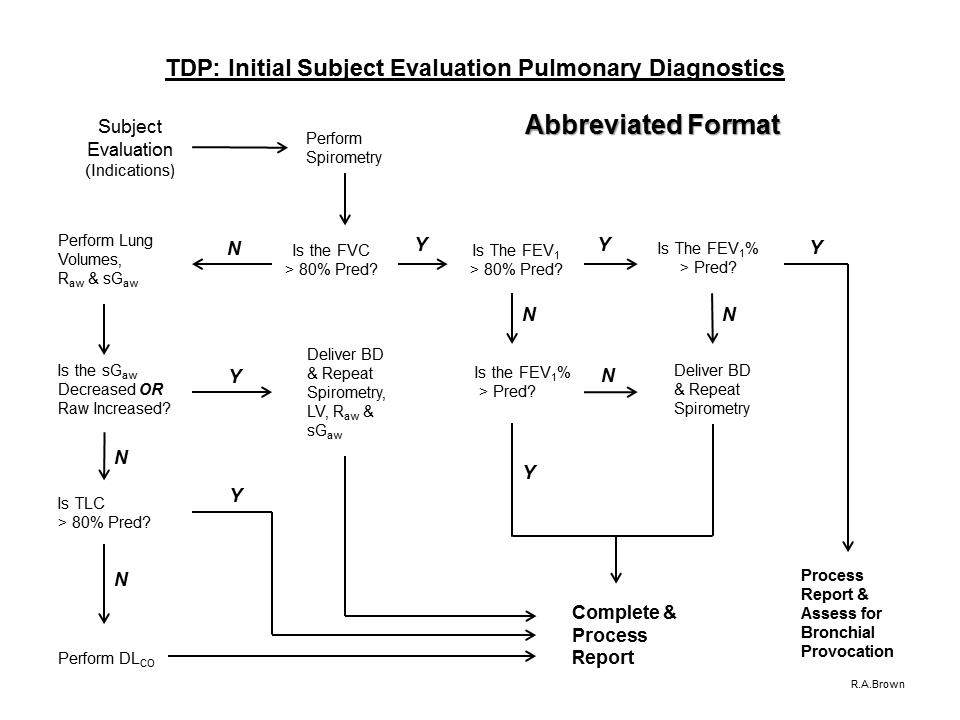
6. If airflow limitation is not present on baseline Spirometry and the FVC value is > 80% predicted (or > ULN) and the subject is symptomatic, proceed with the evaluation process and perform a Flow-Volume Loop:
A. If the Inspiratory Limb is abnormal, perform Airway Mechanics and SBN2 to assess the site of obstruction and Process the Report.
B. If the Inspiratory Limb is normal, perform Airway Mechanics.
C. If Airway Mechanics are abnormal, assess response to acute inhalation of a bronchodilator (Spirometry and Airway Mechanics) and Process the Report
D. If Airway Mechanics are normal, perform MVV
E. Perform MVV to assess
1. Respiratory Muscle Weakness:
a. If the MVV is reduced (because of fatigue), perform Static Lung Volumes, ABG and Respiratory Muscle Forces and Process the Report
2. airway hyperreactivity
a. If the MVV is reduced because of possible airway hyperreactivity (as evidenced on the spirogram) assess using the Bronchial Provocation Protocol
F. If the MVV is normal, perform Static Lung Volumes
G. If Static Lung Volumes are abnormal, or a diffusion defect is suspected, perform Diffusing Capacity
H. If Static Lung Volumes are normal, assess degree of unventilated lung (VTG – FRC)
1. if unventilated lung determination is within normal limits, Process the Report
a. Consider further evaluation of symptoms with Bronchial Provocation Protocol and/or Cardiopulmonary Exercise Test (which includes expired gas analysis)
I. If Diffusing Capacity is abnormal, perform ABG with Hemoximetry and Process the Report.
J. If Diffusing Capacity is normal, assess response to acute inhalation of a beta-agonist (if the RV/TLC ratio is elevated) and Process the Report.
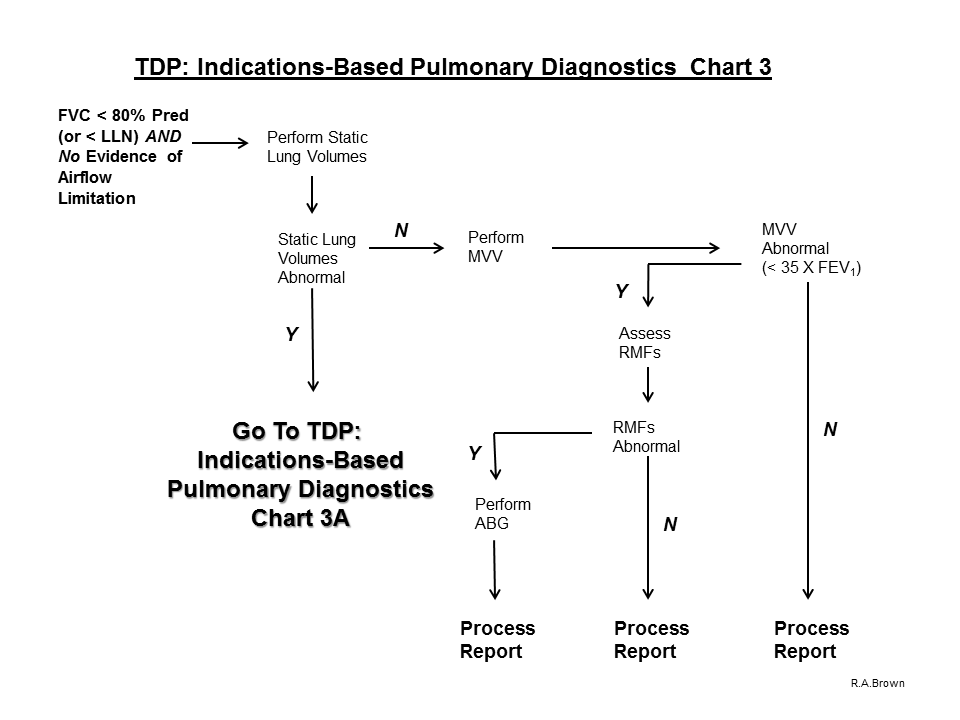
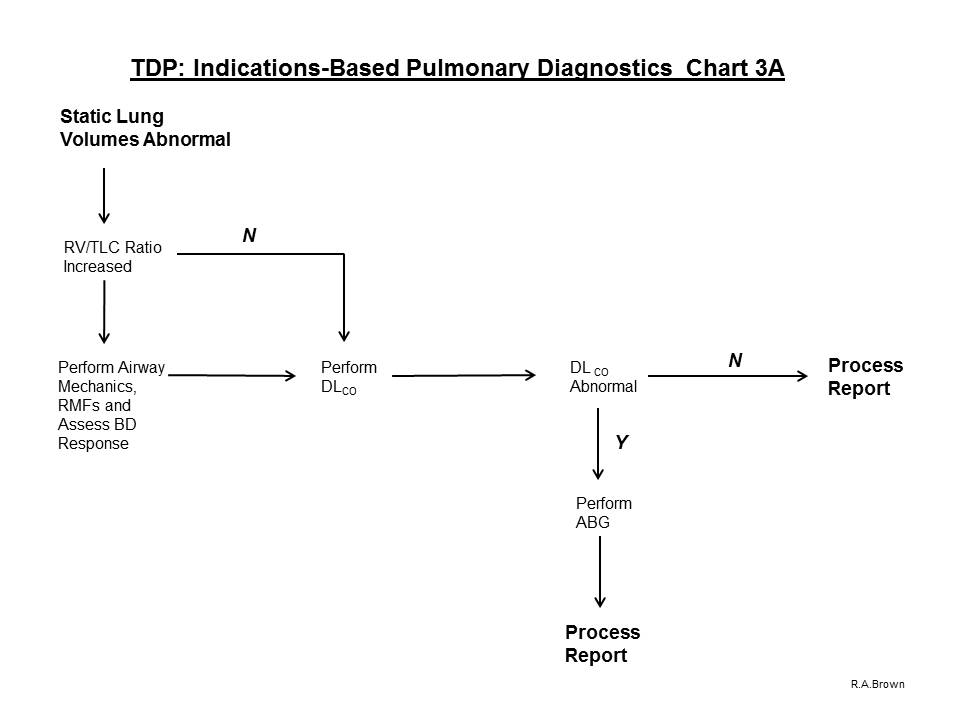
7. If airflow limitation is not present on baseline Spirometry and the FVC value is < 80% predicted (or < LLN), proceed with the evaluation process and perform Static Lung Volumes:
A. If Static Lung Volumes are abnormal:
1. If RV/TLC ratio is increased, perform Airway Mechanics, RMFs and Diffusing Capacity.
a. Prior to dismissing the subject, assess response to acute inhalation of a beta-agonist
2. If RV/TLC ratio is within normal limits, perform Diffusing Capacity
3. If Diffusing Capacity is within normal limits, Process the Report
4. If Diffusing Capacity is abnormal, perform ABG with Hemoximetry and Process the Report
B. If Static Lung Volumes are normal, perform MVV to assess Respiratory Muscle Weakness
1. If the MVV is within acceptable range (FEV1 X 35), Process the Report
2. If the MVV is < predicted minimum (FEV1 X 35) with acceptable effort, assess Respiratory Muscle Forces
3. If Respiratory Muscle Forces fall within the acceptable lung volume adjusted ranges, Process the Report
4. If Respiratory Muscle Forces are abnormal (i.e., below the lung volume adjusted ranges), perform ABG with Hemoximetry and Process the Report.
VIII. GUIDELINES / WARNINGS:
1. Monitor subject’s heart rate (before, during and after) beta-agonist administration. The need to change medication may be indicated by:
A. A heart rate greater than 120 bpm, or if a pulse increase of 20 bpm occurs with bronchodilator medication. Technologist statement must appear on the final report.
B. Patient is receiving noncardioselective beta-blocker therapy.
2. After repeated forced expiratory efforts, worsening of some pulmonary function parameters (e.g., FEV1, instantaneous flows, and airway mechanics) may indicate airway hyperreactivity.
NOTE: This may spontaneously subside, or may require treatment with beta-agonist.
3. Although rare, syncopal episodes do occur with forced expiratory maneuvers.
4. There is the possibility of transmission of airborne diseases either from the technologist to the subject or from the subject to the technologist.
5. Frank hemoptysis warrants immediate discontinuance of testing.
A. A significantly elevated DLCO value may be an early warning of intrapulmonic hemorrhage6.
6. Universal Precautions must be followed with all blood and blood tinged articles9.
7. Technologist test “Quality statements” must accompany all final reports.
A. Technologist should never report values which do not reflect the patient’s true pulmonary physiology.
1) The Technologist needs to provide clear documentation as to why results were not reported. For example, “Despite repeated instructions and demonstrations, the subject either could not, or would not, perform the specified test(s) in an acceptable and reproducible manner. Because of this no data are being reported at this time.”
IX. TECHNOLOGIST RESPONSIBILITY:
1. The INDICATIONS-BASED PULMONARY DIAGNOSTICS: INITIAL SUBJECT EVALUATION protocol will be used to evaluate all subjects referred for testing to the Pulmonary Diagnostic Services.
2. Immediately notify the requesting physician if subject experiences an untoward event during testing (e.g., Vaso-vagal response) or if arterial blood gas results fall within the “Panic Value / Physician Alert”10 range(s).
3. Inform Pulmonary Diagnostic Services Directors (Medical and Technical) about physicians who are repeatedly reluctant to comply with PDT recommendations to alter requested procedures to those that are more appropriate.
A. As a component of the Pulmonary Diagnostic Services Quality Assurance program, these recalcitrant ordering physicians may need to be reeducated in this matter.
X. SUPPORTING REFERENCES
1. Ruppel G. Manual of pulmonary function testing, 3rd ed., Mosby Year-Book Publishers, 1992.
2. American Association for Respiratory Care Clinical Practice Guideline: Spirometry. Respir Care 1991;36(12):1414-1417.
3. American Association for Respiratory Care Clinical Practice Guideline: Static Lung Volumes. Respir Care 1994;39:830-836.
4. American Association for Respiratory Care Clinical Practice Guideline: Single-Breath Diffusing Capacity. Respir Care 1993;38(5):511-515.
5. American Association for Respiratory Care Clinical Practice Guideline: In-vitro pH-Blood Gas Analysis and Hemoximetry. Respir Care 1993;38(5):505-510.
5A. American Association for Respiratory Care Clinical Practice Guideline: Sampling for Arterial Blood Gas Analysis. Respir Care 1992;37(8):913-917.
6. American Association for Respiratory Care Clinical Practice Guideline: Body Plethysmography. Respir Care 1994;39:1184-1190.
7. American Association for Respiratory Care Clinical Practice Guideline: Bronchial Provocation. Respir Care 1992; 37:902-906.
8. Centers for Disease Control. Guidelines for preventing the transmission of tuberculosis in health care settings with special focus on HIV-related issues. MMWR 1990;39:1-29.
9. Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in health care settings. MMWR 1988;37:377-388.
10. Department of Health and Human Services Health Care Financing Administration Public Health Service 42 CFR part 405, et al. Clinical Laboratory Improvement Amendments of 1988; Final Rule. Federal Register Friday February 28, 1992.
==========================================================================================================
TDP: INDICATIONS-BASED PULMONARY DIAGNOSTICS: INITIAL SUBJECT EVALUATION FLOW CHARTS:
Go to Bronchial Provocation TDP at: https://pftlabresources.com/tdp-bronchial-provocation-bronchoprovocation/