TDP: BRONCHIAL PROVOCATION (BRONCHOPROVOCATION)
I. PROTOCOL: BRONCHIAL PROVOCATION (BRONCHOPROVOCATION)
II. PURPOSE: To maintain continuity and assure appropriateness of physician orders for Bronchial Provocation, as performed in the Pulmonary Diagnostic Laboratories. The Pulmonary Diagnostic Technologist (PDT) will assess each request for Bronchial Provocation testing, with regard to the subject’s diagnosis, past medical history, and “baseline” pulmonary diagnostic test results. If indicated, the PDT will initiate the most appropriate protocol.
III. SUBJECT TYPE: All subjects referred to the Pulmonary Diagnostic Services for evaluation of bronchial hyperreactivity.
IV. TESTING AREAS: Adult and Pediatric Pulmonary Diagnostic Laboratories.
V. EQUIPMENT AND FORM NEEDED:
1. Calibrated (with verified calibration) Pulmonary Diagnostic Equipment: Spirometer, plethysmograph, dosimeter (gas source and high quality updraft nebulizer), treadmill (or electronically braked bicycle ergometer).
2. Frigid-air generator and gas source.
3. “Rescue” medications and related equipment.
4. Physician order (written or electronically generated) or completed Physician Request for Pulmonary Function Testing; “Bronchoprovocation”.
5. “TDP: Bronchial Provocation”.
6. “Bronchoprovocation Study: Abbreviated Protocol” report1, or “Bronchoprovocation Study: Standard Protocol” report or “Exercise-Induced Bronchoconstriction study with Frigid-Air Inhalation” report.
VI. OVERVIEW: Physician Request / Order for “Bronchoprovocation”

VII. PROTOCOL: The following guidelines will be followed when assessing appropriateness of physician requests for, and the selection and performance of, the most suitable Bronchial Provocation study and are indicated by the PDT Assessment Protocol.
1. The following conditions are accepted indications for Pulmonary Diagnostic Testing2:
A. To determine the presence and extent of lung disease or abnormality of lung function
B. To determine the extent of disability due to abnormal lung function.
C. To determine the type and progression of the disease or lesion.
D. To determine a course of therapy in treatment of the particular lesion.
2. The selection of the appropriate Bronchial Provocation Protocol is based upon the following3,4,5:
A. Indications for Bronchial Provocation include the need to:
1) diagnose or confirm a diagnosis of airway hyperreactivity (asthma)
2) follow changes in airway hyperresponsiveness
3) document the severity of bronchial hyperresponsiveness
4) determine who is at risk in the military or workplace
5) establish a control or baseline prior to a series of environmental or occupational exposures.
B. Pulmonary Diagnostic Tests available include (but are not necessarily limited to):
1) Spirometry
a. with forced inspiratory limb (at baseline) to assess, quantify, and rule-out the presence of upper airway dysfunction
2. Plethysmography
a. Airway Mechanics (Raw, sGaw)
b. Volume of Thoracic Gas (VTG)
3. The following guidelines will be utilized in the evaluation and selection of the appropriate Bronchial Provocation Protocol based upon the diagnosis or medical history:
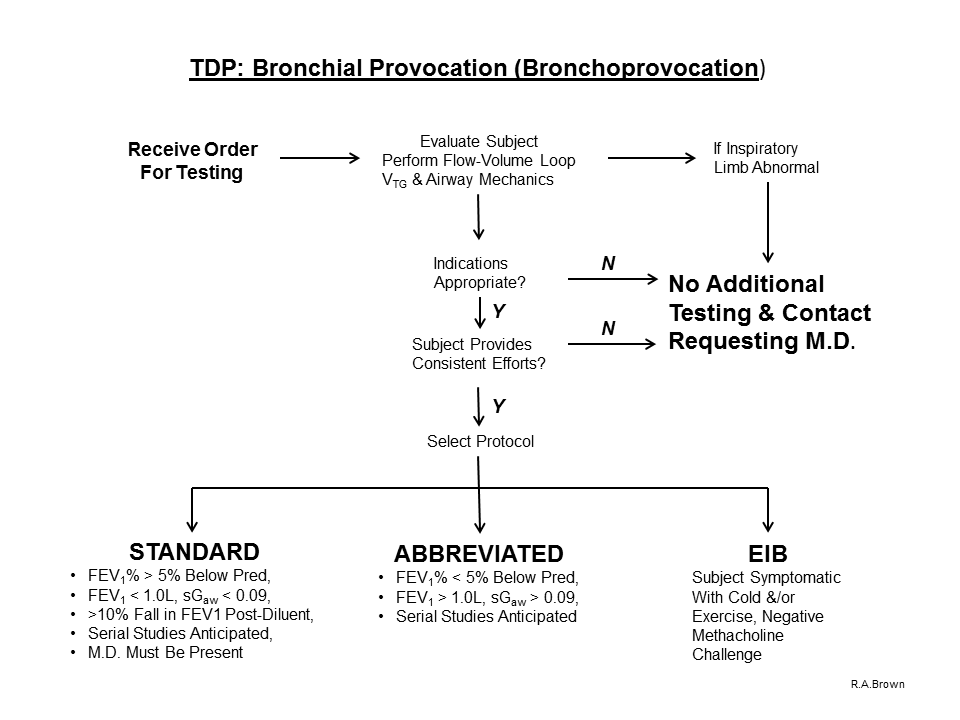
A. Indications for Pulmonary Diagnostic testing employing the “Bronchoprovocation Study: Abbreviated Protocol” (Spirometry [with baseline Flow-Volume Loop], VTG and Airway Mechanics) include, but are not limited to
1) assessment of general, non-specific, airway hyperresponsiveness by inhaling Methacholine diluent solution (placebo), followed by 1 and 4 inhalations of Methacholine at concentrations of 5.0 and 25.0 mg/mL respectively.
2) if there is no evidence or suspicion of “Severe Airway Hyperreactivity” (based upon previous Bronchoprovocation test results, past medical history including the diagnosis of asthma, or recent upper-or-lower respiratory tract infection)
3) if the FEV1 is greater than 1.0 liter
4) if the FEV1 / FVC ratio is greater than 70%
5) if the sGaw is greater than 0.09
6) if the FEV1 did not fall greater than 10% after diluent inhalation
**NOTE: This protocol should NOT be performed if criteria 3,4,5 are not met.
B. Indications for Pulmonary Diagnostic testing employing the “Bronchoprovocation Study: Standard Protocol” (Spirometry [with baseline Flow-Volume Loop], VTG and Airway Mechanics) include, but are not limited to
1) assessment of general, non-specific, airway hyperresponsiveness by inhaling Methacholine diluent solution (placebo), followed by 5 inhalations of Methacholine at doubling concentrations beginning with 0.625 mg/ml and concluding with 25.0 mg/ml.
2) if there is evidence or suspicion of “Severe Airway Hyperreactivity” (based upon previous Bronchoprovocation test results, past medical history including the diagnosis of asthma)
3) if the FEV1 is less than 1.0 liter
4) if the FEV1 / FVC ratio is less than 70%
5) if the sGaw is less than 0.09
6) if the FEV1 fell greater than 10% after diluent inhalation
7) if serial measurements are going to be performed
**NOTE: Because of the possibility of a subject responding to relatively small concentrations of provocative agent, or if criteria 3,4,5 are present and/or if the subject has a confirmed diagnosis of asthma, a physician must be aware of this procedure and be responsive if summoned.
VIII. GUIDELINES / WARNINGS:
1. Unless being used for evaluating effectiveness of therapeutic intervention, failure to withhold the following medications may affect the bronchial provocation test results. These medications should be withheld for the prescribed periods of3:
A. Beta2-adrenergic aerosols – 12 hours
B. Anticholinergic aerosols – 12 hours
C. Disodium cromoglycate – 8 hours
D. Oral beta2-adrenergic agonists – 12 hours
E. H1-receptor antagonists – 48 hours
F. Antihistamines – 72-96 hours
G. Inhaled or oral corticosteroids have been shown to decrease bronchial responsiveness
H. Non-cardioselective beta-blockers may increase bronchial responsiveness.
2. Other factors which may influence test results include2:
A. ingestion of cola drinks, chocolate and other agents containing caffeine or theobromines
B. Smoking
C. specific antigen exposure within the previous week
D. exposure to high atmospheric pollution levels within the previous week
E. pregnancy
F. subjects having recent upper-and-lower tract respiratory infections, sarcoidosis, cystic fibrosis and asthma will commonly exhibit significant bronchial hyperreactivity to minimal concentrations of provocative agent
3. Testing should be postponed if the subject is unable to at least meet ATS recommendations for acceptable spirometry performance.
4. The following untoward reactions may occur during any portion of the bronchial provocation3
A. Bronchoconstriction, hyperinflation, severe coughing (rescue medications and emergency equipment should be close at hand);
B. Hazards associated with Spirometry, such as dizziness, light-headedness, chest pain6;
C. Potential exposure of Technologists to provocative substances.
5. There is the possibility of transmission of airborne diseases either from the Technologist to the client or from the client to the Technologist7.
6. Frank hemoptysis warrants immediate discontinuance of testing.
7. Universal Precautions must be followed with all blood and blood tinged articles8.
8. Technologist “Quality Statements” must accompany all final reports.
A. Technologists should never report values which do not reflect the subject’s true pulmonary physiology.
IX. TECHNOLOGIST RESPONSIBILITY:
1. The TDP: Bronchial Provocation Protocol guidelines will be used to evaluate and re-evaluate all subjects referred for Bronchoprovocation testing to the Pulmonary Diagnostic Services.
2. Immediately notify the requesting physician if the client experiences any untoward event during testing.
3. Inform Pulmonary Diagnostic Services Directors (Medical and Technical) about referring physicians who are repeatedly reluctant to comply with PDT recommendations to alter requested procedures to those that are more appropriate.
A. As a component of the Pulmonary Diagnostic Services Quality Assurance program, these recalcitrant referring physicians may need to be reeducated in this matter.
X. RELATED PROTOCOLS
1. TDP: Assessment of Physician Request for Pulmonary Diagnostic Testing.
XI. SUPPORTING REFERENCES
1. American Association for Respiratory Care. Form of the Month: Bronchoprovocation Protocol Form. AARC Times November 1993:70-71.
2. Ruppel G. Manual of pulmonary function testing, 3rd ed., Mosby Year-Book Publishers, 1992.
3. American Association for Respiratory Care Clinical Practice Guideline: Bronchial Provocation. Respir Care 1992; 37(8):902-906.
4. European Society for Respiratory Physiology (SECPR), Working Group on Bronchial Hyperreactivity. Guidelines for standardization of bronchial challenges with (nonspecific) bronchoconstricting agents. Bull Eur Physiopathol Respir 1983;19:495-514.
5. Subcommittee on Bronchial Inhalation Challenges. Guidelines for bronchial inhalation challenges with pharmacologic and antigenic agents. ATS News 1980; Spring:11-19.
6. American Association for Respiratory Care Clinical Practice Guideline: Spirometry. Respir Care 1991;36(12):1414-1417.
7. Centers for Disease Control. Guidelines for preventing the transmission of tuberculosis in health care settings with special focus on HIV-related issues. MMWR 1990;39:1-29.
8. Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, Hepatitis B virus, and other bloodborne pathogens in health care settings. MMWR 1988;37:377-388.
==========================================================================================================
TDP: BRONCHIAL PROVOCATION FLOW CHART
Go to Infant / Toddler Testing TDP at: https://pftlabresources.com/tdp-guideline-evaluation-of-physician-requests-for-infant-toddler-pulmonary-diagnostic-testing/