I’m assuming, since you’re reading this, that you currently provide some level of Pulmonary Diagnostic procedures. That being said, do you consider yourself as a “Tech” or as a “Technologist”? I believe there is a difference in the terms. Hopefully the following example might clarify that statement.
A 64 y.o. male (inpatient), life-long farmer, is referred for testing as part of his preoperative assessment for a (R) great toe amputation due to a crush injury. His past medical history is unremarkable except for a 23 pack year smoking history (quit at age 41). Requested preoperative pulmonary diagnostic procedures are for “Complete Studies” (i.e., Spirometry with bronchodilator, MVV, Airway Mechanics, Lung Volumes, Diffusing Capacity and resting, room air, Arterial Blood Gases).
My first question is, what would you do in this instance?
* If you are a “Tech”, you would have the subject perform the procedures as requested.
* If you are a “Technologist”, based upon the information provided, you would seek additional information since the requested procedures seem excessive for this subject’s preoperative assessment.
As a Technologist, you are also a diagnostician and provide added value to your organization. You are trained to provide quality driven, cost-effective services to your customers. You are viewed as a valuable resource (the go-to person) and a colleague (a physician-extension and not simply a task-completer) to those HCPs that you interact with on professional matters.
Therefore, you would search further into the patient’s PMH and try to assess the rationale for all the requested procedures. If no compelling additional data is readily available, you would not hesitate to contact the requesting physician and suggest that, for this visit to the Pulmonary Diagnostic Laboratory, the more appropriate “Preoperative Evaluation” studies would be Spirometry (with bronchodilator [BD] if evidence of airflow limitation), MVV (as a compliance indicator) & if the other tests are abnormal, resting ABG (with Hemoximetry and SpO2 [assuming the SpO2 device manufacturer is standardized within the institution]).
Note: Going this extra step provides and demonstrates value to you (you’re a consultant), the customers (the patient and the requesting physician*, who has been enlightened) and the organization (by reducing costs). It also highlights that you are quality driven by providing the most appropriate service to your customers.
* If indicated, based upon the derived initial pulmonary diagnostic data, additional testing may be warranted at a later date.
My second question is, how did you arrive to the conclusion that the original battery of requested procedures (in the preceding example) might be excessive and that the ones you suggested might be more appropriate?
Hopefully your response would be a combination of evidence-based information and vast clinical and/or laboratory experience.
This is where well-constructed Technologist Driven Protocols (TDPs) can come into play. TDPs can provide a roadmap for decision making on the flow of testing based upon the client’s Diagnosis, Medical History, and/or from initial Pulmonary Diagnostic data. TDPs that are well-designed, and validated, add value to your facility and to the organization by reducing procedure variability and improving the quality of service delivery.
The TDPs contained in this section were for the more commonly provided Pulmonary Diagnostic procedures, from the early-to-mid 1990s, and are designed largely from published (evidence-based) information. After the design was finalized and approved by the Pulmonary Quality Assurance (QA) committee, prior to full implementation, each TDP was monitored and amended* for approximately a year as part of the Pulmonary Diagnostic Service QA/CQI program.
The information from Focus Area 1, “Indications for Pulmonary Diagnostic Testing / Summary of Services” was used to develop, and begin validation of, these Technologist Driven Protocols.
* Note: The PDCA Cycle was used initially, but was subsequently switched to the PDSA Cycle: see Implementation Science section for definition of terms.
FYI: The term, “Technologist Driven Protocols” (TDPs), may be controversial as it implies too much onus being placed on the Pulmonary Diagnostic Technologist. Unless TDP is currently utilized in your laboratory setting, an alternate term to consider would be, “Patient-Focused Pulmonary Diagnostic Assessment Plans“. This term is more neutral in tone and may be more readily accepted (especially by decision makers if you are developing and describing the initial TDP planning phase).
TDP Examples:
These TDPs are provided as informational only as recommendations have changed since their inception. However, they might be useful as a guide when developing, evaluating and implementing well designed, individualized, TDPs for a given institution.
Note: You’ll notice the absence of decision symbols; this is intentional. The decision tree is self-evident and symbol inclusion would not add value to the TDP flow.
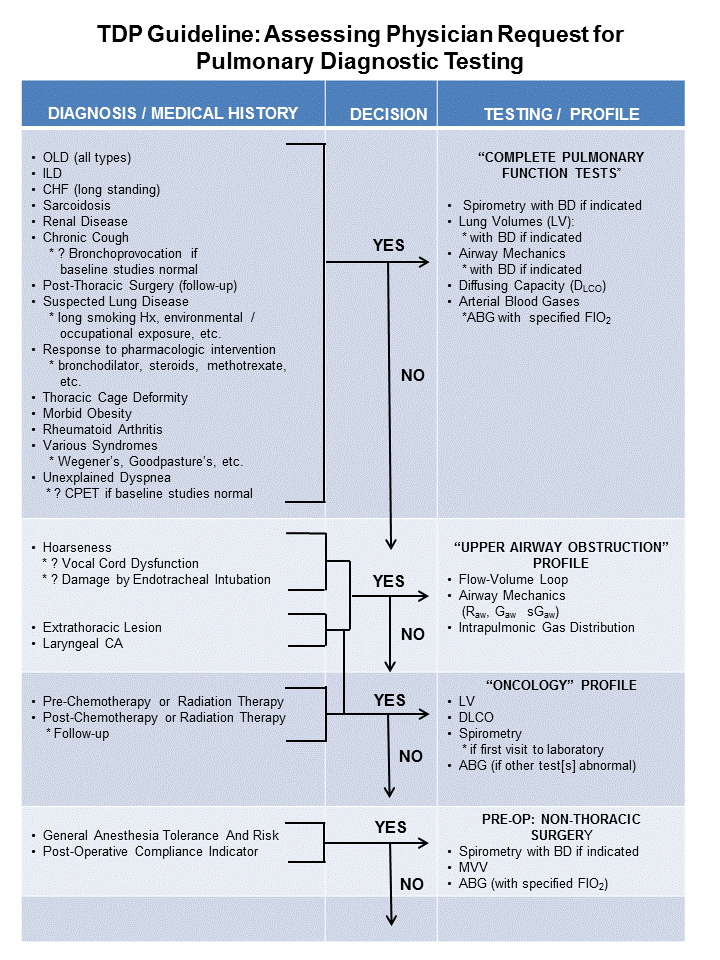
A) The “Assessment of Physician Request for Pulmonary Diagnostic Testing” TDP was first fully instituted and published in 1991. The Pulmonary Diagnostic Services request form (electronic and paper) listed individual procedures, or “evaluate patient”. A number of tests were also grouped into “Profiles” in an effort to assist the requesting physician to have a clear understanding of what services might be most appropriate for his/her patients, based upon their medical history.
- Utilizing Diagnosis &/or Medical History, this TDP aligned the appropriate Testing Profile for the subject.
- A copy of the flowchart (hard copy & laminated small index card size for quick reference) was also included in the “Indications for Pulmonary Diagnostic Testing” information packet previously described.
B) The “Indications-Based Pulmonary Diagnostics: Initial Subject Evaluation” TDP is incorporated by the Pulmonary Diagnostic Technologist (PDT) to assess each testing request with regard to diagnosis, past medical history, or subject symptoms.
The Protocol is well defined and supported by medical literature; it was developed during the evolution and subsequent publication of the listed AARC CPGs, as well as expert opinion.
Note: The flow may initially seemed complex. However, after a couple of run-throughs, the process flow became clearer and easier to apply in practice.
C) The “Bronchial Provocation (Bronchoprovocation)” TDP can be stand-alone, or used in conjunction with the “Indications- Based Pulmonary Diagnostics: Initial Subject Evaluation” TDP. Based upon initial subject testing data, three different challenge methods could be incorporated.
Again, the Protocol is well defined, supported by medical literature and the process/flow is straight forward. More recently published Bronchoprovocation recommendations might be indicated versus what was used for the development and implementation of this TDP.
D) The “Guideline: Evaluation of Physician Requests for Infant / Toddler Pulmonary Diagnostic Tests” TDP is incorporated by the Pulmonary Diagnostic Technologist (PDT) to assess each testing request with regard to diagnosis, past medical history, or subject treatment/intervention.
The Protocol is well defined, supported by medical literature and can be used as a stand-alone process utilizing four different testing protocols.
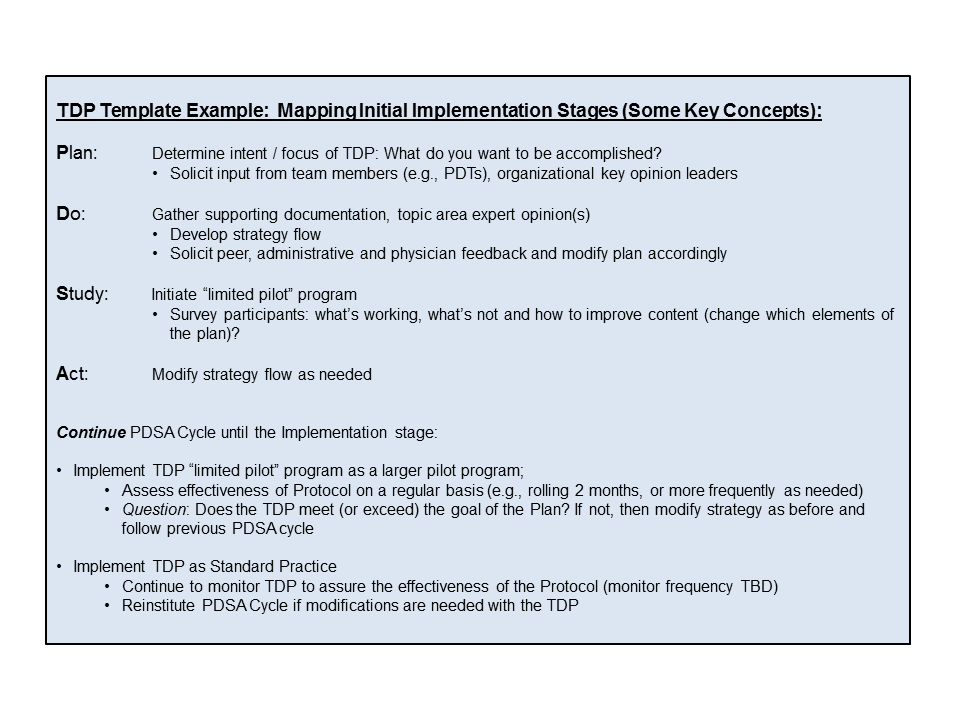
TDP Template (Example):
This following template provides some key Technologist Driven Protocol implementation stages. It is provided as an example only, as it is not all encompassing with regard to how to plan for, or what can be contained within, a particular TDP.
Just a heads-up that before investing a lot of time and energy into developing TDPs, it is probably best to pause and assess your organization’s willingness to be open to change (“organizational readiness”; see Implementation Science section). Realistically, there are many individuals who are reluctant to accept change in the work environment. Going from “the way we’ve always done this” to something more formalized like a TDP, may be inconceivable by some. Therefore, it’s probably worth it to develop a compelling outline focusing on the potential benefits of implementing TDPs and presenting this information to organizational key decision makers. It may also be beneficial to garner early input from laboratory co-workers. Doing so allows them to have a clear understanding of the project intentions, to be part of the development process and hopefully have early buy-in of the project.
TDP Development Process
Data suggests that quality initiatives are generally more successful if they are developed using a team-based approach versus a top-down approach. The TDP development process is in line with this thinking. The rationale is that the more understanding that decision makers have regarding the development and implementation of TDPs, the more likely that they will be supportive of the initiative.
This does not mean that all team members need to be involved with every single aspect of the TDP development. However, all team members do need to be regularly apprised of TDP progress; be it positive or negative. By keeping them in the loop, they will be part of the process and the solution if additional guidance is needed if / when roadblocks are encountered.
**Note: Be patient when detailing your focused outline to decision makers; they may have competing priorities and, unlike you, may not immediately appreciate the importance of incorporating TDPs as part of quality service delivery
While not comprehensive, the following is a potential listing, and role, of some TDP Team Members:
- Organization Administration: Understands the intentions of the project; to elevate the quality of delivered services and potentially reduce institutional costs
- Pulmonary Diagnostic Laboratory Medical Director: The laboratory ambassador who will support the initiative with other HCPs. Will also be the go-to person for potential MD 1-on-1 discussions if there is push back.
- Department of Quality: Has a good understanding of process development / implementation and would be an excellent resource.
- Nursing: Representation from Pulmonary Specialty Outpatient Clinic as they may need to interface with potential clients and provide a brief description of Pulmonary Diagnostic Laboratory testing procedures.
- Pulmonary Diagnostic Technologist(s): Absolutely necessary to be active participants of the entire process, as they are the ones who will be the TDP implementers and ultimately the end-users. Active participation, regarding input and decision making, should enhance buy-in and embrace/own the project.
Well-designed, evidence and expert opinion based TDPs can improve the quality of delivered services by standardizing the approach to client evaluation, reduce inappropriate testing (improve the client/patient experience) and provide value to the organization.
** In my opinion, it is imperative to provide the rationale behind TDP development. Simply developing a flow-chart, without supporting documentation, is only part of the picture and subject to criticism regarding the validity of the TDP.
NOTE: The “ATS Pulmonary Function Laboratory Management and Procedure Manual 3rd Edition” contains referenced Indications and Contraindications for each procedure listed. This manual could be a reasonable resource for determining what supporting evidence might be useful when developing your laboratory specific TDPs. The “ATS Pulmonary Function Laboratory Management and Procedure Manual” can be found at: https://store.thoracic.org/product/index.php?id=a1u33000000ye1TAAQ .
Hopefully this overview has been of assistance as you develop, and/or modify, your Technologist Driven Protocols (TDPs). Remember, this is a process and not a race. There may be more than one iteration of the Protocol before being suitable for full implementation. Incorporating Implementation Science concepts into the development, evaluation and execution of TDPs should be the keys to success to providing a viable and sustainable program.
Please let me know if you need any clarification of the Technologist Driven Protocol Focus Area. This is an extremely important aspect of Pulmonary Diagnostic Laboratory Services and I want you to be successful.
Best O’ Luck!
Bob
Go to Assessment of Physician Requests TDP at: https://pftlabresources.com/tdp-assessment-of-physician-request-for-pulmonary-diagnostic-testing/